Our Offerings
As the world’s largest company for contract development and manufacturing, our Pharma Biotech & Nutrition segment is recognized for its reliable, high-quality services, regulatory track record, global footprint, innovative technology platforms and extensive experience.
Our vision is to enable our customers to meet some of the greatest challenges in patient treatment. Our broad capabilities span across biologics, small molecules (including highly potent active pharmaceutical ingredients such as cytotoxins), bioconjugates, cell and gene technology, and live biotherapeutics. We manage projects from pre-clinical stage through to commercialization, from established therapeutics to advanced personalized medicines, and our expertise covers both drug substance and drug product.
>730
Preclinical and Clinical Small1 and Large2 Molecules
>310
Commercial Small1 and Large3 Molecules
>200
Billion Capsules Produced4
1 Including active pharmaceutical ingredients (API), highly potent API (HPAPI) and dosage form and delivery systems
2 Including mammalian and microbial, cell & gene therapy products, applied protein services and drug product services
3 Including mammalian and microbial and cell & gene therapy products
4 Including pharma and nutritional hard capsules
In February 2019, we changed our structure, combining Pharma & Biotech and Consumer Health & Nutrition into the Lonza Pharma Biotech & Nutrition (LPBN) segment. With this structure, we can leverage our innovation programs and technology platforms across the nutrition-pharma spectrum, most importantly in the pharma and nutritional capsules businesses.
In 2019, the LPBN segment comprised the following offerings:
CDMO service businesses:
Product businesses:
Personal Perspectives
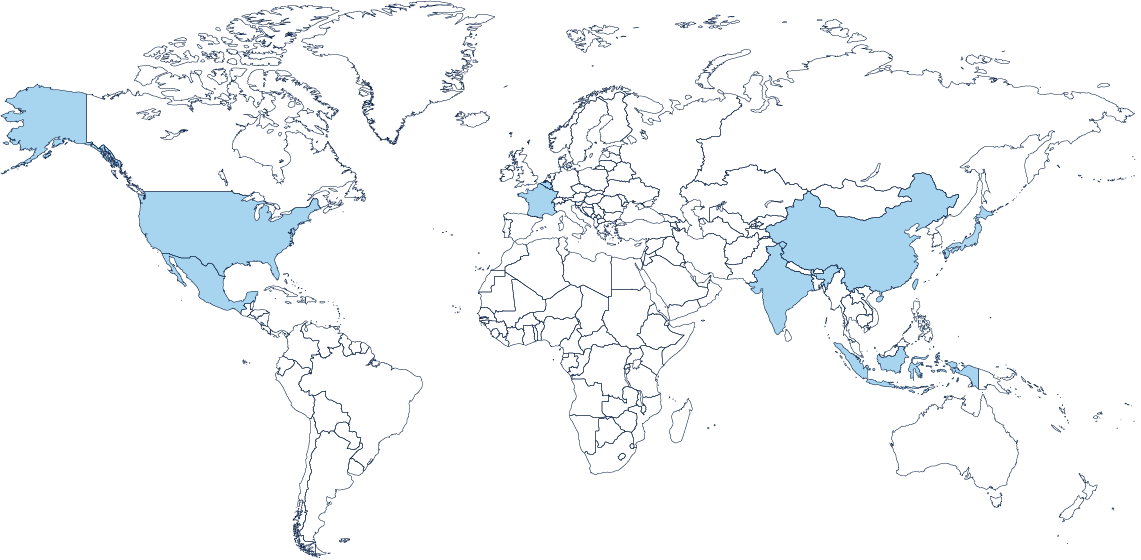
Our Global Footprint
With 37 sites across three continents and 11,148 employees, we capitalize on our global footprint and have the flexibility to address regional and even local marketplace needs.
Portsmouth, USA
Mammalian
- Commercial manufacturing
Hayward, USA
Mammalian
- Clinical manufacturing
Visp, Switzerland
Bioconjugates
- Clinical development & manufacturing
- Commercial manufacturing
Mammalian2
- Clinical development & manufacturing
- Commercial manufacturing
Microbial
- Clinical development & manufacturing
- Commercial manufacturing
Basel/Stein, Switzerland
Drug product services
- Clinical & commercial development
- Clinical & commercial manufacturing1
Cambridge, UK
Pre-clinical candidate risk assessment
- Manufacturability & immunogenicity
- Non-GMP research product supply
Slough, UK
Mammalian
- Clinical development & manufacturing
Porriño, Spain
Mammalian
- Commercial manufacturing
Guangzhou, China2
Mammalian
- Clinical development & manufacturing
Tuas, Singapore
Mammalian
- Clinical development & manufacturing
- Commercial manufacturing
1 cGMP Sterile manufacturing
2 Capabilities to become available from 2020
Portsmouth, USA
Cell & gene technologies
- Clinical & commercial manufacturing
Houston, USA (including El Rio)3
Cell & gene technologies (including viral vector manufacturing)
- Clinical & commercial development & manufacturing
Kingston, Canada
Cell & gene technologies
- Clinical development
Geleen, Netherlands (including Maastricht)3
Cell & gene technologies
- Clinical & commercial development & manufacturing
Tuas, Singapore
Cell & gene technologies
- Clinical development & manufacturing
Tokyo, Japan4
Cell & gene technologies
- Clinical & commercial development & manufacturing
3 Locations connected to another site
4 Facility owned and operated by Nikon CeLL innovation Co. Ltd. under Nikon-Lonza partnership
Quakertown, USA
Particle engineering and drug products
- Dosage forms & delivery solutions
Tampa, USA
Particle engineering and drug products
- Dosage forms & delivery solutions
Bend, USA
Particle engineering and drug products
- Dosage forms & delivery solutions
Visp, Switzerland
Drug substance
- API development & manufacturing
Monteggio, Switzerland
Particle engineering and drug products
- Dosage forms & delivery solutions
Ploermel, France
Particle engineering and drug product
- Dosage forms & delivery solutions
Edinburgh, UK
Particle engineering and drug products
- Dosage forms & delivery solutions
Nansha, China
Drug substance
- API development & manufacturing
Greenwood, USA
Pharma & nutritional capsules
Puebla, Mexico
Nutritional capsules
Colmar, France
Pharma & nutritional capsules
Bornem, Belgium (including Komec Helsen)
Pharma & nutritional capsules
Sagamihara, Japan
Pharma & nutritional capsules
Suzhou, China
Pharma & nutritional capsules
Jakarta, Indonesia
Pharma & nutritional capsules
Haryana, India
Pharma & nutritional capsules
Benicia, USA
Nutritional ingredients
Cohasset, USA
Nutritional ingredients
Fort Smith, USA
Nutritional ingredients
Nansha, China
Nutritional ingredients
Walkersville, USA (including Wayne and Salisbury)3
Bioscience
Durham, USA
Bioscience
Rockland, USA
Bioscience
Verviers, Belgium
Bioscience
Cologne, Germany
Bioscience
Copenhagen, Denmark
Bioscience
3Locations connected to another site
Financial Highlights
Lonza Pharma Biotech & Nutrition (LPBN) achieved continued double-digit sales growth above guidance for 2019. The realigned segment includes the nutritional hard capsules business (acquired with Capsugel), as well as a small portfolio of nutritional ingredients and formulation services. We delivered CHF 4.2 billion sales in full-year 2019 and a CORE EBITDA of CHF 1.4 billion while investing in strategic growth projects, a number of which are expected to commence operations from the end of 2020.
Million CHF |
2019 |
Change in % |
2018 Restated1 |
||
|---|---|---|---|---|---|
Sales |
4,167 |
11.0 |
3,755 |
||
CORE EBITDA |
1,371 |
10.0 |
1,246 |
||
Margin in % |
32.9 |
|
33.2 |
||
CORE EBITDA excl. IFRS 16 |
1,347 |
8.1 |
1,246 |
||
Margin in % |
32.3 |
|
33.2 |
||
CORE result from operating activities (EBIT) |
1,125 |
10.3 |
1,020 |
||
Margin in % |
27.0 |
|
27.2 |
||
CORE result from operating activities (EBIT) excl. IFRS 16 |
1,123 |
10.1 |
1,020 |
||
Margin in % |
26.9 |
|
27.2 |
||
|
|||||
Sales
Million CHF
CORE EBITDA
Million CHF
CORE EBITDA Margin
In %
1 Reported pro-forma full-year 2017 financial results include Capsugel full-year 2017 financial results
2 Restated to reflect the 2019 realignment of Lonza’s segments into Pharma Biotech & Nutrition and Specialty Ingredients