Market Trends
Market trends support all four areas in which Lonza Bioscience operates: Therapeutic Cell Culture Media, Research Tools, Testing Solutions and Quality Control Software.
The global media market was estimated in 2017 at USD 1.4 billion with a 10% CAGR over the next 10 years. This market is expected to reach USD 4-5 billion driven by existing biologics (recombinant proteins and vaccines)1.
In the Discovery market, recent developments in Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and non-viral, ex-vivo gene therapy are driving demand for alternative technologies, like nucleofection, whilst new applications in in vitro toxicology and immunotherapy are driving demand for liver cells and immune cells, supporting custom cell biology services.
Several developments and trends drive demand for offerings in the informatics and testing markets. Large Pharma and Biotech companies are actively adopting global, integrated and automated solutions in quality control and manufacturing environments. Traceability and data integrity within current good manufacturing practices (cGMP) is becoming a critical part of the manufacturing and release process for a lot of these companies. With Cell and Gene therapy on the rise, there is an increasing need for cost-effective and flexible IT systems, which can be rapidly deployed to improve decision making, quality and compliance needs.
1 Sources: Meticulous (2018); BCC research (2015); Markets and markets (2015); Seed planning; METI; Kuick Research, Med Market Diligence, Transparency market research, Roots analysis cell therapy manufacturing market (2017–2027)
310
Bioscience Products Filed with Regulatory Agencies
>2,600
Scientific Publications Used a Lonza Bioscience Product
222
Different Primary Cell Types
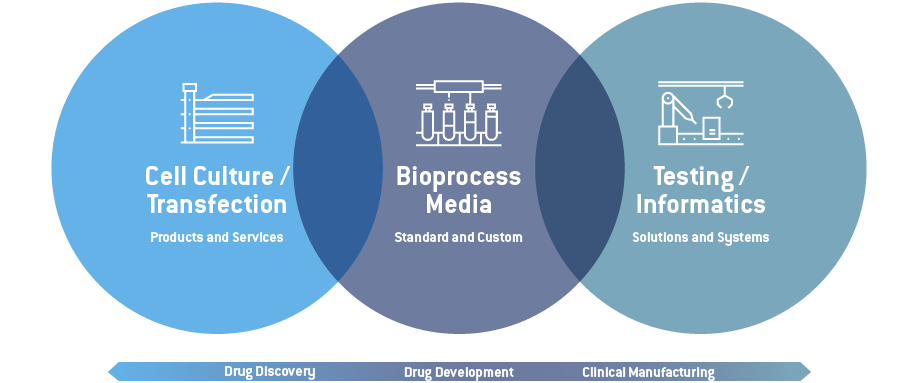
Our Offerings
With our product offerings in media, research tools, testing solutions and quality control software, we help our customers leverage the therapeutic potential for transformational technologies in cell and gene therapy and biologics.
Our offerings provide advanced cell biology and cell engineering solutions, Quality Control (QC), safety testing tools and software. Collectively, these support the life-science industry from discovery through to manufacturing.
We serve customers in academic and government institutions and major biotech and pharmaceutical organizations across the globe. Bioscience customers are discovering, developing and manufacturing critical disease-treating drugs and therapies, and we provide tools to support their activities and ensure the highest degree of patient safety and regulatory compliance. With our product offerings we help our customers leverage the therapeutic potential for transformational technologies in cell and gene therapy and biologics.
Our therapeutic cell culture media are used in the production of therapeutics, including antibodies, antibody drug conjugates (ADCs), vaccines, cell and gene technologies applications and other biologics.
Our Discovery Solutions offer human cell-based tools for basic research, drug-discovery and translational research targeting cardiovascular, respiratory, neurological, metabolic, cancer and other disease areas.
Our Testing Solutions offer endotoxin-detection assays that are applied in pharmaceutical product-release testing, medical device testing and dialysis clinics. They help to ensure the safety of injectable drugs, implantable medical devices and dialysis equipment.
The fully integrated MODATM Software Solutions streamline quality-control processes and offer insight into manufacturing operations, with quick access to management, compliance and trending data.
Bioscience Offerings Overview
Our Global Footprint
Our Bioscience business serves customers across the world with a network of seven key production sites.
In Europe, the team at our Cologne (DE) site is dedicated to the manufacturing and sales of a non-viral transfection method, which enables a more efficient identification of new targets for pharmaceuticals and therapies. Our second site in Verviers (BE) is the European distribution center for Bioscience Solutions research products and produces cell culture media for bio-production, cell and gene therapy and research applications. Our Copenhagen (DK) site specializes in custom manufacturing unique agaroses for chromatography purposes.
In the USA, our Walkersville, MD (USA) site is the North American distribution center for Bioscience Solutions research and as well as producing cell culture media, we also manufacture endotoxin testing reagents, automation and software solutions for the quality control of parenteral drugs, medical services and biomanufacturing products.
Further sites in the USA include Durham, NW (USA), which is a Center of Excellence that specializes in the manufacture of primary cells for research, drug discovery and drug development. In Wayne, MI (USA), we provide software solutions for environmental monitoring and electronic batch recording. In Rockland, ME (USA), we provide life science researchers with state-of-the-art products for use in molecular biology as well as cell based assays for clinical diagnostics, rapid cell health and activity screening and other applications.
Discover More
Additional information about our offerings, such as process development, current good manufacturing practices (cGMP), assay development, analytical and all other related services, is available online. Explore our worldwide sites by location via our 360° Virtual Tours.
Highlights and Initiatives 2019
The Bioscience Solutions business saw increased demand, based on favorable market trends in drug discovery and cell therapy. We are continuing to make progress with operational improvements.
Therapeutic Cell Culture Media
With over 40 years of expertise in media development, we introduced eCHOTM1 Basal Medium and Feed. eCHOTM Medium is a new serum-free, chemically defined, hydrolysate-free and non-animal origin medium to enhance late-stage cell viability, accelerating protein purification and allowing for the production of a consistently increased amount of proteins per cell.
1 Chinese hamster ovary (CHO)
Our leading TheraPEAKTM X-VIVOTM Cell Culture Media have seen strong growth in 2019, supporting the CAR-T process and other treatments under development in the cell therapy market. Our Bioscience Solutions and Cell & Gene Technologies groups are joining forces to promote a full offering in cell and gene therapy, helping customers to successfully navigate the path from early discovery to commercialization, and get new treatments to patients faster.
Bioscience Solutions and our Mammalian and Microbial Development and Manufacturing groups are intensifying their collaboration in order to help customers in the biologics market using our GS-CHOTM Media as an integral part of our GS Gene Expression System®.
Research Tools
Bioscience continues to shape the cell and gene therapy market, with integrated solutions and our cell biology expertise both positioned to support cGMP cell processing workflows, including those based on Lonza’s Nucleofector® Transfection Technology. Market adoption of the 4D-Nucleofector® LV Transfection Device increased as we accelerated efforts to support cGMP workflow requirements and establish Nucleofector® Technology as the standard for non-viral transfection in cell and gene therapy applications.
In 2019, we developed a line of high-quality cryopreserved pooled donor suspension hepatocytes, DonorPlexTM Hepatocytes. In addition, we added Verified for SpheroidsTM Human Hepatocytes, which are pre-screened for their ability to promote spheroid formation in 3-dimensional cell culture platforms, supporting toxicology, disease modelling and Drug Metabolism and Pharmacokinetics (DMPK) studies.
Customers working on immunology applications can take advantage of the largest available portfolio of immune cells, due to the private label partnership between Lonza Bioscience and AllCells. This partnership expands our broad offering of hematopoietic cells supporting an array of applications in drug discovery, toxicity testing, cell therapy and personalized medicine.
To meet specific, individual research application needs, we have expanded our CellBio Services, a comprehensive portfolio of unique, custom solutions. Researchers across pharmaceutical and contract manufacturing organizations can now choose from an extensive range of services, including cell line expansion and banking, media production, cell isolation, cell characterization, transfection services, and three-dimensional (3D) cell-culture services.
Testing Solutions
Due to sustained interest from new and existing customers, we continue to expand our global availability of the world’s first fully automated, plate-based robotic solution PyroTecTM PRO Robotic Solution for endotoxin testing. The platform marks a milestone in endotoxin detection, allowing pharmaceutical manufacturers to replace manual, error-prone processes with a fully automated solution, integrating instruments, endotoxin reagents and software offering quick time-to-result. In addition, our PyroGeneTM Recombinant Factor C Assay, is gaining traction in the market, as regulatory authorities have started accepting the assay as an alternative method for endotoxin testing.
Quality Control Software
During the reporting year, the Bioscience business experienced strong interest in the MODATM Platform. We launched the next-generation electronic batch record execution solution, MODA-ESTM Software Platform, designed to provide a cost-effective, easy to use solution to batch record challenges, by consolidating and managing batch and quality data generated by non- or semi-automated manufacturing processes.